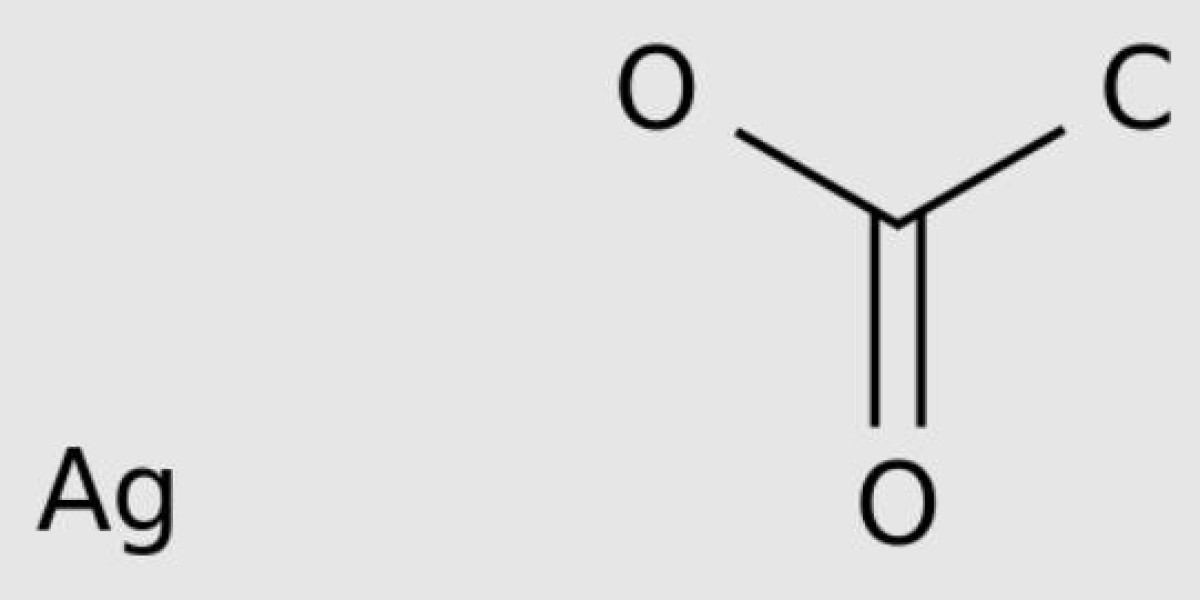
Silver acetate is composed of the elements carbon, hydrogen, silver and oxygen. Carbon is a non-metal in group 14 of the periodic table. Its atomic number is 6, denoted by the symbol C. Hydrogen is the lightest, colorless, odorless, tasteless, and flammable gas. Its atomic number is 1, denoted by the symbol H. Silver is a soft, white, shiny transition metal found in Group 11 of the periodic table. Its atomic number is 47, denoted by the symbol Ag. Oxygen is a highly reactive non-metal in Group 16 of the periodic table. Its atomic number is 8, denoted by the symbol O.
Silver acetate is a white crystalline compound with the formula C2H3AgO2. It has a slightly sour smell. Silver acetate is a photosensitive compound, that is, sensitive to light. It is a moderately water-soluble source of crystalline silver that decomposes to silver oxide when heated. Silver acetate is used in the medical field for the manufacture of anti-smoking drugs, and also for the preparation of reflective and conductive silver-coated polymer films. Other names for silver acetate are silver monoacetate and silver acetate.
Preparation of silver acetate
Silver acetate is prepared by the reaction of acetic acid and silver carbonate at 45-60°C. In addition to silver acetate compounds, water and carbon dioxide are also produced.
2CH3CO2H+Ag2CO3⇢ 2C2H3AgO2+H2O+CO2
Silver acetate is prepared by the reaction of silver nitrate with sodium acetate solution.
AgNO3+CH3COONa⇢ CH3COOAg+NaNO3
Physical properties of silver acetate
Silver acetate appears as a white crystalline compound.
The molecular weight of silver acetate is 166.912g/mol.
Silver acetate has a boiling point of 220°C. It has a density of 3.26 g/cm3.
Silver acetate is insoluble in water.