Discover postsExplore captivating content and diverse perspectives on our Discover page. Uncover fresh ideas and engage in meaningful conversations
Property24 helps Australians sell or rent properties affordably, giving full control over listings, pricing, and communication while saving on agent fees and reaching serious buyers or tenants easily. #realestate #property24
Curso de Pizzero Profesional: Aprende el Arte de la Pizza Domina las técnicas de la pizza italiana con un curso de pizzero profesional. Aprende masas, salsas, toppings y secretos de chefs expertos. Visítanos - https://escuelapizzeria.es/
Upgrade your style with our stylish Rijac work bags for women(link opens in new tab/window)! Lightweight and easy-to-handle ladies work bag which safely holds all the essentials for daily chores, without compromising in style. Rijac has just what you need. Our work handbag comes with a zipper which keeps your essentials safe while keeping you stylish and organized from morning to night. Check our collection and elevate your everyday look! Link - https://www.rijac.com/collections/work-bags-women
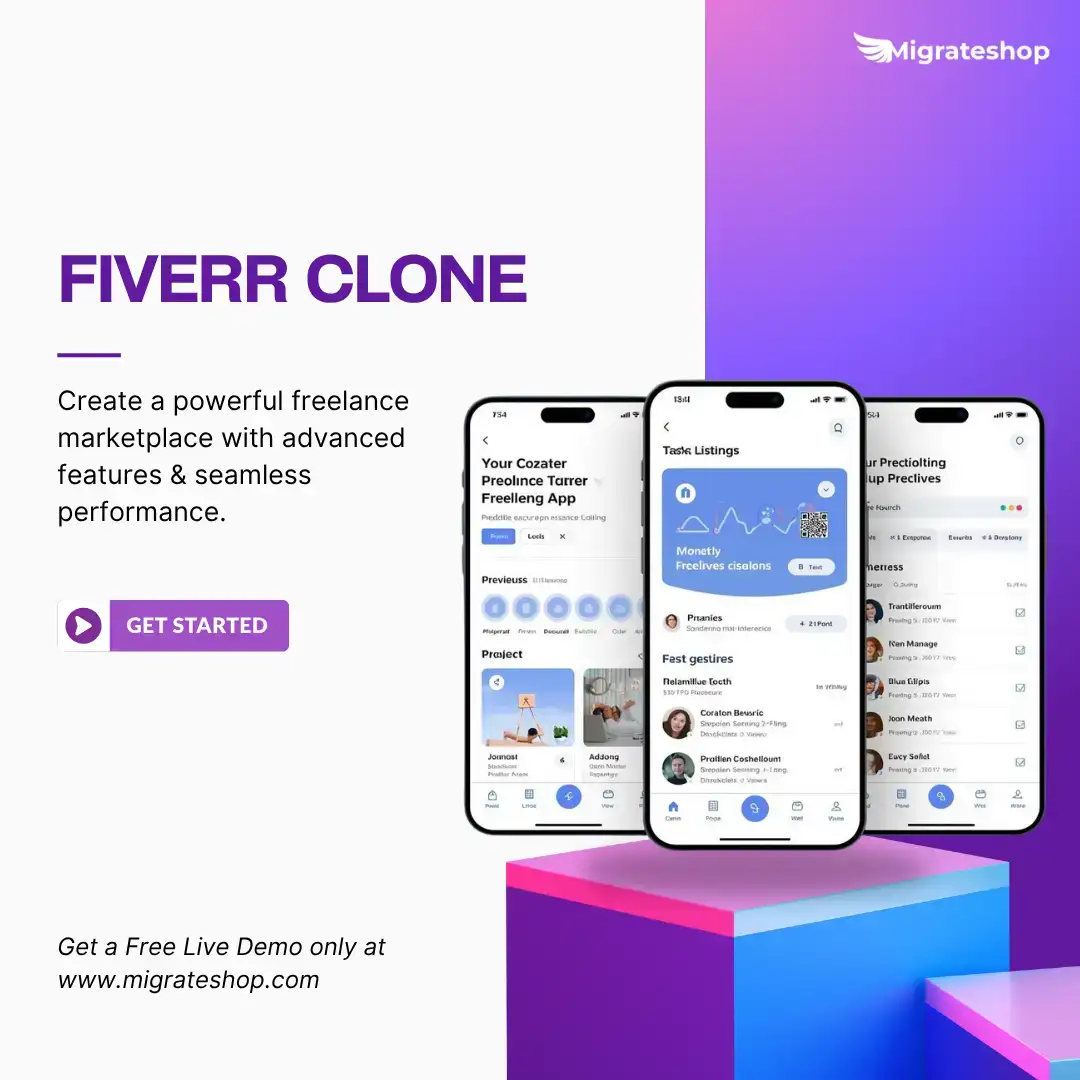
Turn your freelance business idea into reality with our Fiverr Clone. Launch your own freelance marketplace platform where professionals and clients connect seamlessly. Manage projects, payments, and communication — all in one ready-made Fiverr clone app. Explore more: https://migrateshop.com/fiverr-clone/ #fiverrclone #fiverrclonescript #fiverrcloneapp #migrateshop #freelancemarketplacescript #seo #trending #usa #business #clonescript
Keeping your home spotless in a busy city like Toronto isn’t always easy. Between long work hours, commutes, and family life, deep cleaning often gets pushed aside. That’s why many homeowners now rely on professionals to handle it. But finding a trusted cleaning company in Toronto one that’s safe, transparent, and reliable isn’t just about who offers the lowest quote. It’s about who genuinely cares for your home and your family’s health. https://crivva.com/article/tru....sted-cleaning-compan #carpetcleaning #carpetcleaningservices