Beryllium hydride can be synthesized by reaction of dimethylberyllium with borane or lithium aluminate 43 or pyrolysis of di-tert-butylberyllium.
As a molecular example, we consider the beryllium hydride molecule BeH2 in the experimental geometry. The Gaussian basis set is the 6-31G basis of the Pople, and the Slater exponent has been obtained by the equation. (21). The two HF valence orbitals (ag and b1u) of this linear molecule are shown in the figure. 4. Note that the cusp correction scheme does not correct the second MO on the Be kernel because the value of that MO is actually zero on that center.
Beryllium forms many borides, among which BeB2. The compound is copper-red in color and has a cubic fluorite structure, a= 4.661A. BeB2 is easily hydrolyzed by dilute acid to produce beryllium hydrogen. The CAS number of BeB2 is 12429-94-6 and the molecular weight is 73.878g/mol. This red solid melts at >1970°C. Other beryllium borides are insoluble in acids. They don't melt below 1500°C. When hydrochloric acid reacts with beryllium boride, it is found that the yield of tetraborane (B4H10) is higher than that of magnesium boride.
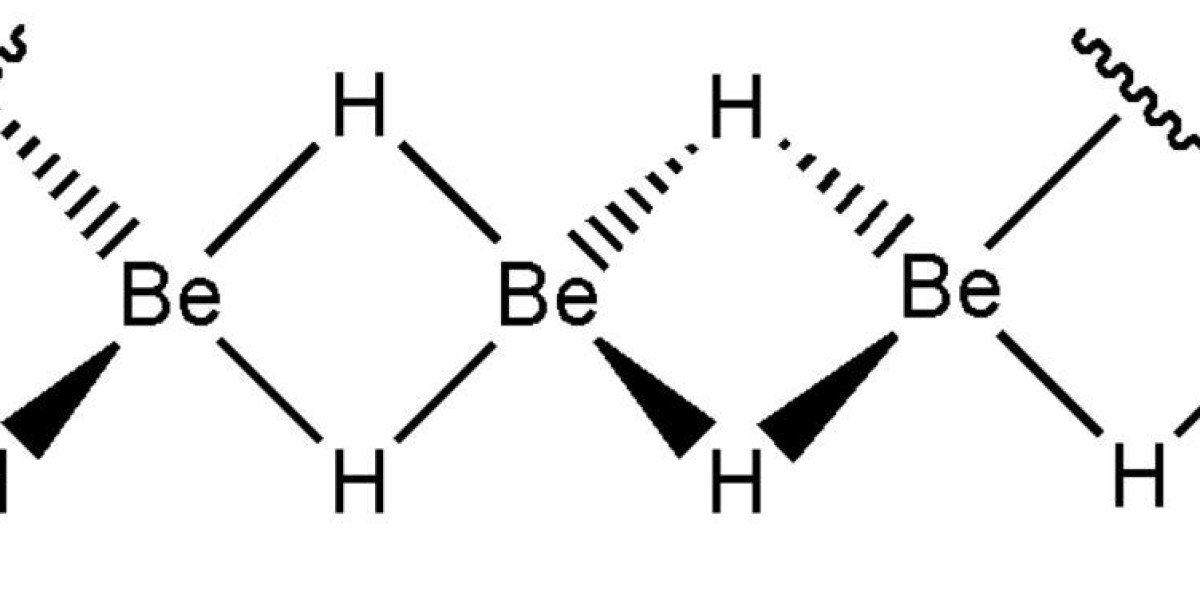
BeH2 was first synthesized in 1951 by the reaction of dimethyl beryllium Be (CH3) 2 with lithium aluminum hydride LiAlH4. The pyrolysis of di-tert-butylberyllium {be (C (CH3) 3) 2} at 210°C also results in a purest form of BeH2. The purest beryllium hydride is obtained by the reaction of triphenylphosphine (i.e. PPh3) with beryllium borohydride Be (BH4) 2, as follows:
Be (BH4) 2+2PPh3⇒ 2Ph3PBH3+BeH2
Note that unlike other elements in Group IIA, where hydrides can be prepared by reaction of elements, the reaction of metals with hydrogen to produce beryllium hydride is not yet possible.
 " class="wow_main_float_head_img">
" class="wow_main_float_head_img">







